Shipment Reports
— Comprehensive shipment data to optimize your cold chain
Our advanced shipment data goes beyond simply tracking temperatures—it provides comprehensive oversight of your cargo from start to finish. With greater visibility of your shipment, you can identify any weak points in your cold chain and make proactive decisions to optimize your logistics.
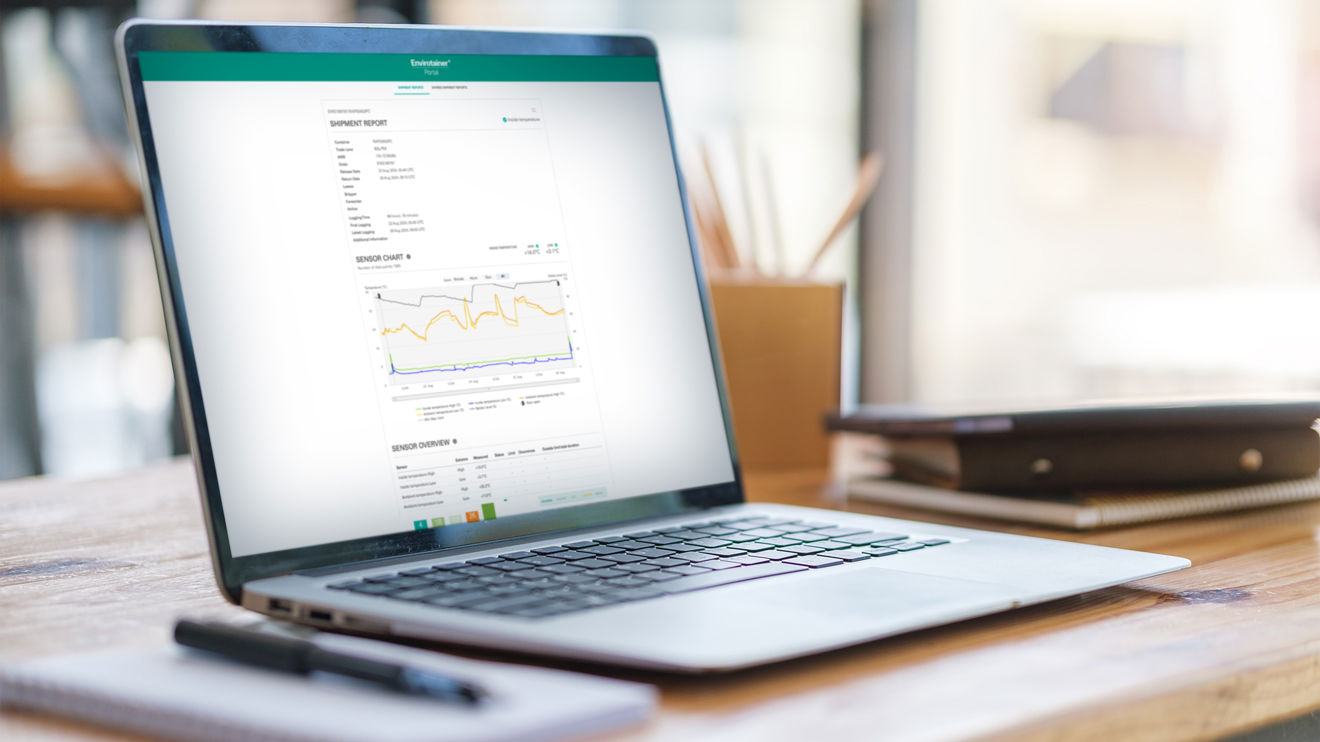
How it works

Industry leading monitoring throughout your shipment.
By continuously monitoring temperatures, as well as battery levels (ULD only) and if the solution has been opened at any point, data sensors will record the condition of your products from origin to destination.
Upon arrival, the shipment data is transferred to our cloud solution which generates a comprehensive report, giving you invaluable insights into the journey your products have taken.
Available in the Envirotainer Portal within 48 hours upon return to an Envirotainer station, your Shipment Report will support faster product release and gives you the data you need to optimize your processes.
Key Benefits
Increasing the visibility of your products journey to support effective collaboration and faster product release.
Supporting faster product release
Comprehensive report data allows you to accelerate your quality assurance processes, reducing the need for additional testing upon arrival. This minimizes delays, and streamlines the approval process with regulatory bodies, enabling quicker distribution and timely delivery to patients.
View Sample Report
Enabling effective collaboration
Strengthen collaboration and ensure successful shipments by sharing shipment data with all key stakeholders, as standard, in the Envirotainer Portal. By leveraging data-driven insights, stakeholders can identify weak points and make informed decisions to optimize processes and ensure that your shipments are consistently handled with the utmost precision and care.

Ensuring data integrity
Our procedures are designed to meet the current regulatory requirements for the Pharmaceutical Industry, including 21 CFR 210/211, EU GMP Part I, ICH Q7R1, EU GDP, and WHO GDP standards. This compliance ensures accurate documentation of the precise conditions your pharmaceuticals experience during transport, eliminating the risk of delays due to data inconsistencies.
Learn moreGet started with the Envirotainer Portal
The Envirotainer portal revolutionizes how you manage your logistics, offering a single, user-friendly hub for all your needs. Accessible from any device, at any time, it simplifies your workflows, allowing you to efficiently handle order management, financials, live monitoring and more.


